Laws of Thermodynamics.
The first law of thermodynamics is a more general statement of the law of conservation of energy. In order to illustrate the second law of thermodynamics we use a "pipeline diagram" to illustrate heat transfer processes, engines and refrigerators, in which the amount of heat, H, or work, W, done is represented as a "stream" whose width is proportional to the amount of energy being transfered.
The processes shown below are possible ones, and are observed in nature.
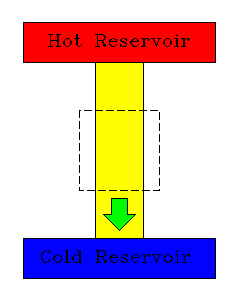 |
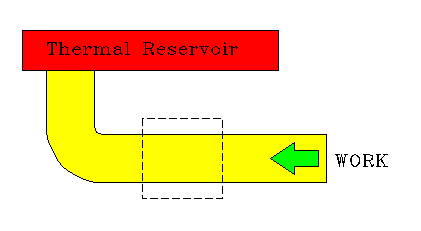 |
| Normal transfer from hot to cold reservoir. |
Conversion of work to heat. |
|---|
But the reverse processes are never seen in nature. The next two diagrams illustrate processes that are impossible in nature. Clausius and Kelvin used these imagined engines in their statements of the second law of thermodynamics.
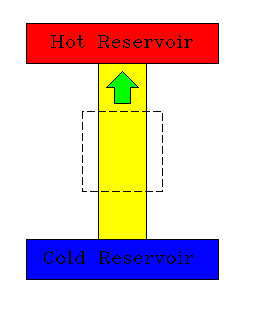 |
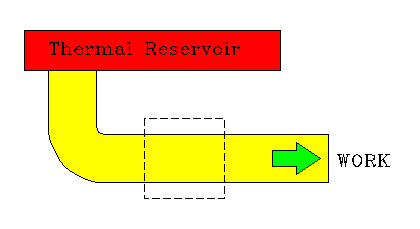 |
| Clausius: No engine can transfer heat from a cold to a hot reservoir without input of work. | Kelvin: No engine can extract heat from a thermal reservoir and convert it entirely to work. |
|---|
Both heat engines and refrigerators may be constructed. While the diagrams (below) look as if you could simply reverse the engine to make a refrigerator, mechanical and other engineering considerations may render this impossible. You cannot turn your automobile engine into a refrigerator in this way. (Many engines have processes that would be difficult or impossible to reverse. To reverse such an engine you'd have to convert the exhaust back into liquid gasoline.) Some refrigerator designs allow reversal of the flow of their liquid refrigerant, and then may be used transfer some heat from a cold reservoir to a warmer one. These are sold as "heat pumps". But in the two modes of operation, their efficiency (as defined above) will be quite different.
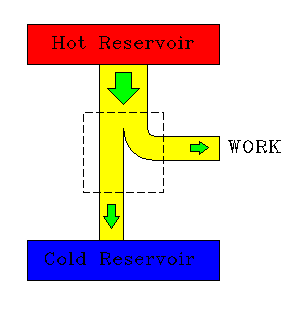 |
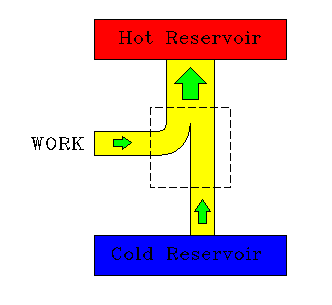 |
| A heat engine. | A refrigerator. |
|---|
The efficiency of a heat engine is defined as:
efficiency = W/Qh = (Qh - Qc)/Qh
The subscripts "h" and "c" label the hot and cold reservoirs.
The performance of a refrgerator is often expressed by a different kind of efficiency rating, one more closely related to the "economic efficiency" of operating it. This will not be used here.
As we noted, most engines are not practical to run in reverse. But we can imagine an idealized reversible engine, one in which all mechanical and thermodynamic processes in the engine are strictly reversible. Such an engine may be operated as a refrigerator with the same ratios of heat to work, and therefore the same efficiency rating in either mode of operation. We refer to them simply as "reversible engines".
A reversible engine and refrigerator may be coupled together so that the work output of an engine provides the necessary work input to a refrigerator. This is a "do-nothing" arrangement, for the two together have no work input and no work output. The system as a whole, including both reservoirs, is not violating any laws of thermodynamics. However, one must ask what maintains the "flow". There won't be any flow of energy. This example shows the limittions of this graphic idealization of heat engines. It shows only energy transfers, without taking account of the inherent inefficiencies of real engines. Something like imagining a frictionless flywheel.
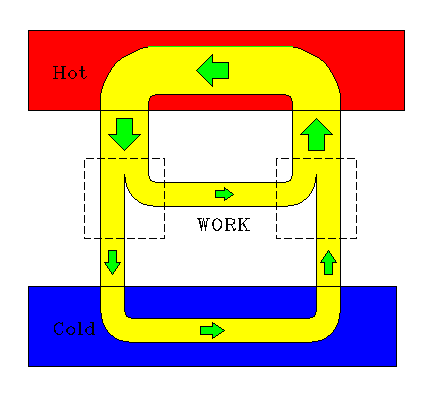 |
| A heat engine drives a refrigerator. |
|---|
We may easily show that the two statements of the second law are identical. We imagine a device that would violate Kelvin's statement of the law (dotted square), and use its output to drive a refrigerator (dotted circle). The result is a composite machine that violates Clausius' statement of the law by transfering heat from a cold to hot reservoir without doing anything else.
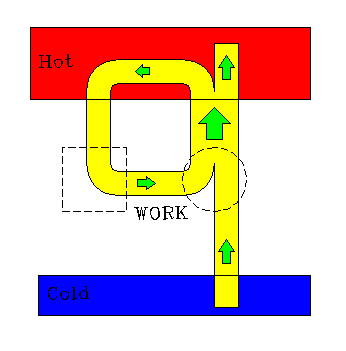 |
| Anti-Kelvin device drives refrigerator. |
|---|
Now try an anti-Clausius machine coupled to a heat engine, and show that in this way you create a device that violates Kelvin's statement of the second law.
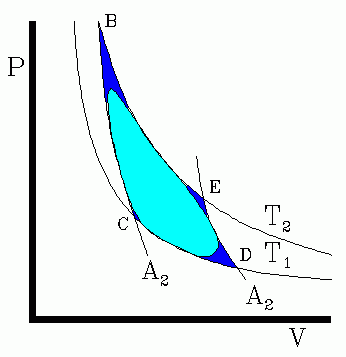
Sadi Carnot proposed a hypothetical engine that was more efficient than any real engine can possibly be. It uses an ideal gas as a working medium, operates infinitessimally slowly (to eliminate inertial effects, and, of course, has zero friction and other dissipative processes. It consists of a cycle of processes BCED, bounded by two isostherms T1 and T2 and two adiabats A1 and A2. These are shown on a pressure-volume diagram. Along path BC and ED, thanks to perfect insulation, the gas expands and contracts along two adiabatic lines (no change in internal energy along these paths). Along path BE the gas expands at constant temperature, taking in heat from the hot reservoir at temperature T2. Along path DC the gas is compressed, giving up some heat to the lower constant temperature reservoir T1.
The Carnot engine is also reversible, for the processes of its operation are individually reversible. Its efficiency can be calculated from the properties of the ideal gas. We omit the derivation here. The result is:
Carnot efficiency = (Th - Tc)/Th
The Carnot engine's efficiency is less than one, obviously. Its efficiency approaches 1 when the temperature of the cold reservoir approaches absolute zero, or when the temperature of the hot reservoir goes to infinity.
The work output of the engine is proportional to the area bounded by BCED. Real engines operating between the same two temperatures necessarily move through their cycles faster, so the abrupt discontinuities of the Carnot engine's graph cannot be attained. Such a real engine's cycle is illustrated by the light blue area, and is necessarily a smaller area than that of the Carnot engine, therefore the work output is smaller.
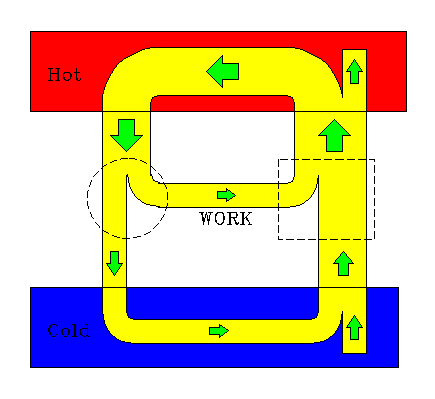
You may say "Why can't some other kind of engine be more efficient than the Carnot engine?" Suppose someone claims to have such a super-efficient engine. We can show that such an engine must necessarily violate the second law of thermodynamics. Operate the Carnot engine in reverse as a refrigerator, which we can always do, for the Carnot engine is (by definition) reversible. We represent the Carnot engine with the dotted square symbol (on the right side of the diagram). Now operate that super-efficient engine between the same reservoirs. It is represented with the dotted circle symbol (on the left side of the diagram). Let the work output of the Carnot engine drive the refrigerator.
Note carefully the relative widths of the pipelines. The "super-efficient" engine on the left converts about half its input heat to work. The Carnot engine on the right has a work/heat ratio of less than one half (about 0.4 in the diagram). We have drawn these pipeline streams to show greater efficiency for the hypothetical super-efficient engine. But we have drawn an impossible situation, for the engine uses only a part of the heat the Carnot engine delivers to the hot reservoir, and the Carnot engine removes the same amount of heat from the cold reservoir. This is indicated by the pipelines shown inside the reservoirs. The net result is the transfer of heat from a cold to a hot reservoir, in violation of Clausius' version of the Second law.
 |
| A super-efficient engine (left) and a Carnot refrigerator (right) violating the second law. |
|---|
Let's see what we are up against. The Carnot engine operating betwen reservoirs at 311 K and 811 K has an efficiency of 62%. (Check this result.) A steam turbine operating between these same temperatures has an efficiency of 40%. Gasoline engines operate betwen the ambient air temperature and the temperature of the gasoline burning in the cylinder, say between 289 K and 1944 K. The Carnot efficency for these temperatures is 85%. But current gasoline engines achieve only about 30% efficiency, so the engineers have a long way to go. Even a perfect gasoline burning engine of this sort would still waste 15% of the fuel energy used, but we are presently wasting more than 2/3 of the energy of the fuel consumed. And that doesn't count the inefficiency of the vehicle itself, the energy lost to roadway friction and air drag.
There's much political balyhoo now about hydrogen-burning engines, and the fact that they are "clean", producing very little pollution at the point of burning. This would be helpful in crowded urban areas. But producing and extracting and liquifying the hydrogen, and transporting it to the hydrogen fueling stations would produce pollution, too. It would just be "elsewhere" from the point of use. And the extraction of hydrogen from water requires energy, from fuels of some sort. To determine the relative energy-efficiency of systems, we must look at all energy costs of extraction and refining of the fuel, and distribution of it to the point of use. We should also consider in this math the cost of disposing of the waste, cleaning up the pollution, and the economic costs of all of the side-effects. In the past we have not included these in the equation.
If all the hot-air produced by politicians could be turned into useful work, it might help some.
Would greater fuel efficiency solve our energy resource problems? Hardly. As someone once observed, if you build more highways, more people will own cars and more will drive and more will drive greater distances on average. If autos become more fuel-efficient, people will drive more and many will drive larger cars, fewer people will car-pool, and we will still be consuming fuel resources at a pace which will equal or exceed population growth. It's a losing game, driven by two things: the rapid pace of population growth, and a culture based on the notion that excessive consumption is a good and necessary thing to "sustain the economy". One can't help being reminded of the historic "bubbles" of the 17th and 18th century, and wonder when this bubble will burst.
- — Donald E. Simanek, March 29, 2005.
Go to the next chapter, Kinetic Theory of Gases. Newtonian mechanics of gases.
Return to the contents page of A Brief Course in Classical Mechanics.
Return to Donald Simanek's front page.