G-1 ABSOLUTE ZERO
Mercury manometer with Charles' law bulb attachment, metal can large enough to surround the bulb, bunsen burner, crushed ice, dry ice.
2. THEORY:
When the volume of a gas sample is kept constant, the relation between the pressure and temperature of the sample is found, from the general gas law, to be P/T = constant, where P and T are both expressed in absolute units. This relation is one of Charles' laws, represented by a straight line graph passing through absolute zero. Of course this law only holds for the gaseous state. Real gases liquify before absolute zero is reached. However, if the slope of the line is accurately established at higher temperatures, its slope may be extended with a ruler to intercept at absolute zero for V = 0.
PRECAUTION: Mercury is an environmental pollutant. Read the posted listing of its hazards and precautions for its use. Its large mass gives it high inertia, so make all adjustments slowly to avoid spills. Have a mercury spill kit in the lab, and use it promptly if a spill occurs.
3. PROCEDURE:
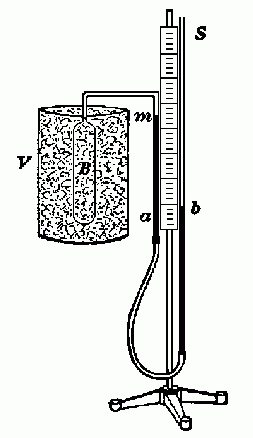 |
| Fig. 1. Air thermometer. [Millikan, Gale and Bishop] |
|---|
To obtain the most accurate data, pressure determinations will be made at three well known, standard temperatures: (a) the steam point, (b) the ice point, and (c) the sublimation point of dry ice (solid carbon dioxide). Thus you'll have no need to use a thermometer to measure the temperature of the gas. You will need to read the barometric pressure, for two reasons: (1) the atmospheric pressure must be added to the manometer reading to obtain the absolute pressure of the sample, and (2) it is needed to determine the steam point temperature.
The apparatus consists of a mercury manometer with a glass sidearm and bulb. This arrangement is sometimes called an "air thermometer." The mercury level in the sidearm is kept at an index mark (m), engraved on the glass tube, to ensure a constant volume of gas in the bulb.
The bulb (B) may be surrounded with a vessel (V) containing boiling water to bring the gas to the steam point. Then surrounded it with a mixture of well crushed ice and water for the ice point. Finally, if dry ice is available, mix the crushed dry ice with acetone to ensure good thermal conduction to the bulb, and surround the bulb with the mixture. (The sublimation point of CO2 is -78°C.) If dry ice is not available, the first two fixed points are sufficient to determine absolute zero, but with lower accuracy.
4. ANALYSIS:
The gas law, for constant volume, is P = cT, where T is measured on the absolute, Kelvin, scale. This is a linear relation. Imagine a plot of this, P vs. T, with T expressed in Celsius scale. It's still a straight line plot, which intercept the temperature axis at the Celsius value of absolute zero. Sketch this, to reinforce the idea. c is the slope of the line, c = ΔP/ΔT. Two data points are enough to determine c, then c and one data point are enough to find the intercept.
This gets more interesting when you have measured P at three fixed points of T. You must use all three to determine absolute zero without data cancelation. Examine your method scrupulously to be sure it is valid.
If you have three points, you might want to plot the graph. But, unless the graph is made very large, you won't be able to get a sufficiently good result directly from the graph.
5. QUESTIONS:
To honor the historical roots of physics laboratory instruction, we've taken these questions from Laboratory Physics by Millikan, Gale and Bishop. Ginn and Company, 1914.
(1) What percent error would have been introduced into the result (absolute zero) by an error of half a millimeter in determining the height of the mercury column at the steam point?
(2) If the boiling point of water on the day of your experiment were 99.5° instead of 100°, what percent error would you have introduced into the result by calling it 100°?
(3) At absolute zero are the molecules of the gas in motion? Are the electrons in the atoms at rest? Explain what absolute zero means.
Text © 1997, 2004 by Donald E. Simanek.