PM-2 ARCHIMEDES' PRINCIPLE
To investigate various methods for measuring densities of solids and liquids, making use of Archimedes' principle.
2. APPARATUS:
Micrometer caliper, vernier caliper, two half-meter sticks, overhead triple beam balance with specific gravity platform, double-pan trip scale (optional), overflow can with catch bucket, hydrometers (for light and heavy liquids), specific gravity bottle (pyknometer), 250 ml graduate, various beakers, straight glass tubing, glass U-tube, rubber tubing.
Unknowns:
a) Various metal samples.
b) Light liquid sample (alcohol-water mixture).
c) Heavy liquid sample (salt-water mixture).
3. THEORY:
Read the material on Archimedes' principle, density, and specific gravity, in any textbook.
I. Archimedes' principle: When a body is fully or partly immersed in a liquid, that body experiences an upward force (the buoyant force) equal to the weight of the displaced liquid. The displaced liquid is that volume of liquid equal to the volume of the body below the water surface.
Another principle will be needed in some of the measurement methods.
II. When any two points in the same liquid are at different heights, the pressure at the lower point, p1, and the pressure at the higher point, p2, are related by
p1 - p2 = ρgh
where ρ is the density of the liquid, g is the acceleration due to gravity, and h is the vertical distance between the points. The phrase "in the same liquid" is very important, and means that a path may be drawn between the two points, that path never leaving the liquid.
III.When an experimental situation includes both liquids and gases exerting pressure on each other, one may assume that any two points in the same gas have essentially the same pressure, independent of height. This is because the densities of gases are so much less than densities of liquids, that pressure differences in the gas due to height differences are negligible compared to pressure differences in the liquids.
4. EXPERIMENTAL METHODS:
A. Direct determination of density by mass and volume measure- ment. the mass is easily determined by weighing. Volumes of liquids are easily determined with a graduated cylinder. Volumes of solids of simple shape (spheres, cylinders, cubes) may be determined by direct measurement of their dimensions with rulers or micrometer and vernier calipers.
The only new technique you will need is when the volume of an irregularly shaped solid must be found. Here the overflow can may be used, with a liquid to overflow through the side spout. Fill the can till its overflows. Then attach a string to the solid and lower it into the can until it is fully submerged, catching the overflow liquid in a beaker. Now find the volume of the overflow liquid, by any appropriate method. If that liquid is water, it may simply be weighed, for 1 ml water has volume 1 cm3 and mass of 1 gm.
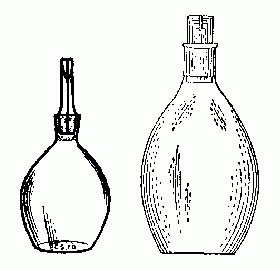 |
| Fig. 1. Pyknometers. |
|---|
B. Specific gravity bottle (pyknometer). This special bottle is used to compare weights of precisely equal volumes of two liquids. The bottle has a special glass stopper with accurately mating ground-glass surfaces. Stoppers are NOT interchangeable between bottles; do not mix them up. The stopper has a hole which allows liquid to overflow as the stopper is inserted, insuring an accurate full fill every time.
The bottle must be weighed dry first. Then weigh it filled with the unknown liquid. Carefully dry it out (an alcohol rinse drys it quickly). Then weigh it filled with water. Dry it again.
C. Manometer methods. These methods use principles II and III to compare the densities of two liquids. Figs. 1 through 3 illustrate several possibilities, and leave it to the student to do the mathematical analysis. The method of Fig. 1 can only be used with non-miscible liquids. In Figs. 2 and 3 the two liquids are separated by air and never mix (if you are careful!). Fig. 2 shows an especially convenient method. The two U-bends are shaped so that a half-meter stick can be nested in each U for direct measurement of the height differences.
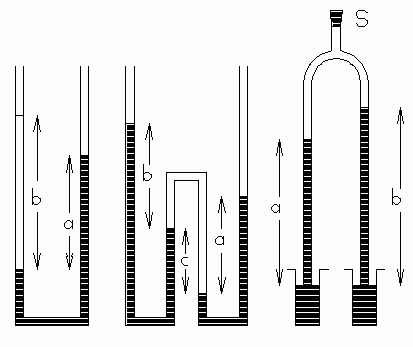 |
| Fig. 2, 3, 4. |
|---|
In Fig. 4 there's a stopcock or cork at S where reduced pressure may be applied to draw up the liquids in the tubes. A "Y" connector with three pieces of rubber tubing and a pinch clamp works well. This method requires measuring sticks which are not harmed by immersion in the fluids.
In all cases a length a is measured for material A and a length b is measured for material B. The formula relating the densities is:
Though this equation is the same for all three cases, the derivation of the equation is different in each case. The student should derive this result for each case, starting from first principles.ρ B b —— = — ρ a A
D. Archimedes' Principle. This principle figures into many of the most accurate and practical density measuring devices. The primary purpose of this experiment is to investigate some of these methods. Other methods (A through C above) may be used to check your results.
5. PROCEDURE:
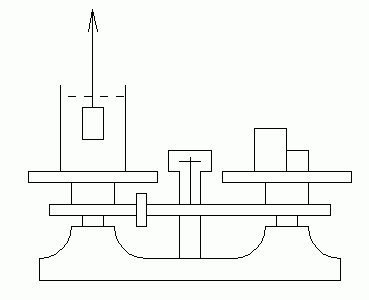 |
| Fig. 5. Double pan trip balance used for density comparisons. |
|---|
(1) Place a beaker of water on a double-pan trip-balance. Balance the instrument's scales. Now what do you think will happen if you were to immerse your finger in the water, without touching the beaker. Analyze the situation and make your predication before you try it, using Archimedes' principle. If this doesn't make sense, consult with your instructor before proceeding.
(2) The specific gravity of brass is 8.4. What is the volume of a 500 gm brass weight (from a weight set)? Predict what would happen if this weight were suspended from a string and lowered into the water on your scales (as in Fig. 5. Predict how much mass would have to be added to or removed from the other pan to rebalance the scales under these conditions. Check your prediction by experiment. You can check your volume calculation by use of an overflow can.
(3) Obtain an unknown solid of irregular shape. You will use the method you have just discovered to determine its density. Weigh the unknown solid in the normal manner. Weigh the beaker with water, balancing the scales. Suspend the unknown solid in the water, and determine how much weight must be added to rebalance the scales.
Analysis: Since the water exerts a buoyant force B upward on the solid, then by Newton's third law the solid exerts force B downward on the water. This unbalances the scales by amount B, and mass having weight B must be added to rebalance them. Thus this added weight equals the buoyant force, and Archimedes' principle allows us to calculate the volume of the solid.
Check your volume measurement with the overflow can, realizing that it won't be as accurate as the Archimedes' principle method.
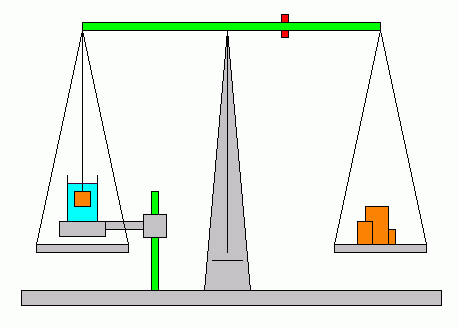 |
| Fig. 6. Overhead double pan balance for density comparisons. |
|---|
(4) Overhead beam balances (Fig. 6) usually have a platform which may be positioned above one pan so that a beaker of liquid can be set on it. This feature is provided specifically for measurements of density using Archimedes' principle.
Suspend the unknown solid object with a string as shown, and balance the scales. Now place the beaker of water on the platform and adjust the platform height until the solid is fully submerged. Rebalance the scales. The change in the scale readings represents the buoyant force. The initial scale reading is just the solid's weight. Calculate the density of the unknown solid from these measurements.
(5) Obtain a beaker of the light unknown liquid, and suspend the unknown solid in it. Using what you have learned, construct a formula for calculation the density of the unknown liquid from just these data: scale reading with unknown solid hanging freely, scale reading with solid immersed in water, scale reading with solid immersed in unknown liquid. The density of the solid will not enter the calculation, and you needn't even know what it is. Find the density of the unknown liquid from you formula. This method is the principle of the standard apparatus known as the Mohr-Westphal balance.
Check this result by use of a hydrometer. Place a large sample of the liquid in the tall graduate, and slowly lower the hydrometer into it until it floats freely. Read the hydrometer scale at the liquid level. Hydrometers often have some strange scales for specialized uses, so you must determine which one represents specific gravity. Many hydrometers are made in Europe, and they often use a comma for a decimal point.
(6) Obtain a rectangular block of wood. Weigh it and measure its dimensions, thereby calculating it volume and density directly. Predict what fraction of its volume would be below water level if it were floated on water (Use Archimedes' principle again). Check your prediction.
(7) A large tank of water is available. Predict how much weight you could place on your wooden block before it will sink. Check your prediction.
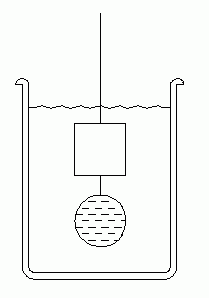 |
| Fig. 7. Method for solids that would float. |
|---|
(8) The basic method of immersion (procedure 3 and 4) is straightforward for measuring density of objects heavier than water. But objects lighter than water would float. A simple modification of procedure is required.
Obtain a cork or piece of wood to use as an "unknown" solid. First weigh it dry. Obtain a metal weight with a hook (perhaps a lead sinker). Suspend the cork from a string (as you did in procedure 3 or 4), and hang the lead weight just below it. Now take two measurements with your scale, one with just the lead weight under water, and one with both lead and cork under water. The difference in these weights is the buoyant force on the cork.
This method obviously works with either experimental arrangement (of procedure 3 or 4), and the metal weight merely serves to keep the cork under water. We don't need to know anything about the weight of the metal sinker.
(9) If time remains you might wish to investigate some of the manometer methods for comparing densities of liquids.
6. THINGS TO THINK ABOUT:
(1) Many books state Archimedes' principle in this way: "The buoyant force on a body is the loss of weight of that body when it is immersed in a liquid."
Now that phrase "loss of weight" ought to worry you a bit. If weight is defined as "the force on an object due to gravity," then the body doesn't "lose" any weight when immersed, the force on it due to gravity is the same whether it is in air, in liquid, or just sitting on the table.
So what is meant by "loss of weight?" Perhaps the above statement of Archimedes' principle assumes that we are using the (standard) method of procedure (4). There we "weigh" the object in air, then "weigh" it in liquid and take the difference in "weights" as read from the balance scale. But the second scale reading is not a "weight" by the usual definition, it is merely the tension in the string supporting the body. The difference in these two tensions is the buoyant force. Since we customarily treat any reading from a balance scale as a "weight reading," that may explain this strange statement of Archimedes' principle.
Some authors have proposed that we should define weight in a different way. They suggest: "'weight' is the force required to support an object at rest in its frame of reference." Defined this way, the weight of an object sitting on the table is mg, as before. The 'weight' of our object immersed in liquid is just the tension of the supporting string, consistent with the statement of Archimedes' principle above. The 'weight' of an astronaut in an orbiting space shuttle is zero. The astronaut is falling just as fast as his space-capsule, and no supporting force is required to keep the astronaut at rest with respect to space shuttle frame of reference.
(2) You probably noticed that all of the methods employing Archimedes' principle with a balance scale required the subtraction of two scale readings. Balances compare forces (weights), but their scales read mass units (grams). If we examine the analysis of procedure (4) we see that this doesn't matter.
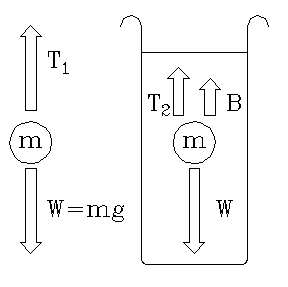 |
| Fig. 8. Forces on a suspended body. ("Loss of weight" in a liquid. |
|---|
Suspended in air: T1 = W = ρsVg
Suspended in liquid: T2 + B = mg
Therefore, T1 - T2 = B
But Archimedes' principle tells us that: B = ρ2Vg
T1 - T2 = ρwVg
T2 ρsVg rs ——————— = ———— = —— = ss T1 - T2 ρwVg rw
Thus the specific gravity of the sample depends on the tensions T2 and T1 of the supporting string. The balance scale directly reads these tensions. But since our formula has a ratio of tensions, they may be measured in any consistent units"even in "gram-weights."
7. QUESTIONS TO BE ANSWERED BEFORE COMING TO LAB:
(The instructor will check these answers before you are allowed to begin the experiment.)
(1) Derive the equation for specific gravity determined by the method of Procedure (4). Work out the random-error propagation equation. Use this to determine how many grams of a brass sample would be needed to obtain its specific gravity to 1%, if the balance scales can read to a precision of 0.1 gram.
(2) A bucket of water is sitting on a bathroom scale. The scale reading is recorded. A block of wood is floated on the water. How much does the scale reading change when the block is added? Now consider pushing the wooden block down carefully with your hand until it is "just" submerged (its top surface is level with the water surface). What determines the bathroom scale reading now? Show how the three scale readings can be used to determine the specific gravity of the wood.
8. QUESTIONS TO BE ANSWERED IN YOUR LAB REPORT:
(3) Will the added weight of the string affect the results of procedure (4)? Will if affect the results of procedure (3) if it were not included in the calculation?
(4) One kilogram of iron and one kilogram of brass are suspended from separate balance scales, each metal submerged fully in water. How do the scale readings compare? Express your answer as a function of the densities of the two materials.
(5) Two overflow cans are filled with different liquids. A wooden block will float in either liquid. Compare the volumes which overflow when a block is floated in each of the overflow cans. Relate this to the densities of the liquids. Also compare the overflow weights.
(6) One cm3 of brass and one cm3 of aluminum are each weighed in air and then in water. Compare their "losses of weight." Relate this to the densities of the two materials.
(7) Precision of reading the hydrometer scale is determined by the spacing of the markings on its stem. The sensitivity can be increased and the marking spacing made larger, by making a hydrometer with a larger bulb and/or smaller stem. Explain why this is so.
(8) In figure 3, what determines the height c? Express your answer as a formula. In view of this, is the figure drawn correctly?
(9) [MMG] Can you see from your analysis any general relation which must always exist between the density of a floating body, the volume of the body, the volume which is beneath the surface and the density of the liquid?
(10) You used a sinker to submerge a piece of cork beneath the water to determine the cork's density. Why was it unnecessary to know the density of the sinker?
(11) The supply room attendant offers you two hydrometers, both appropriate to the range of specific gravities you need to measure. One has a wider diameter stem than the other. Knowing only this fact, which of the two will you choose for the greatest precision?
(12) Use Archimedes' principle to prove the following: "When a body is floating on a liquid, it displaces a weight of liquid equal to its own weight."
Text and diagrams © 1998, 2004 by Donald E. Simanek.