G-2 BOYLE'S LAW
1. THEORY:
Boyle's law, PV = constant, shows how the pressure and volume of a gas are related when a constant quantity of the gas is kept at a constant temperature. This law is a special case of the general gas law, PV = nRT.
PRECAUTION: Mercury is an environmental pollutant. Read the posted listing of its hazards and precautions for its use. Its large mass gives it high inertia, so make all adjustments slowly to avoid spills. Have a mercury spill kit in the lab, and use it promptly if a spill occurs.
2. PROCEDURE:
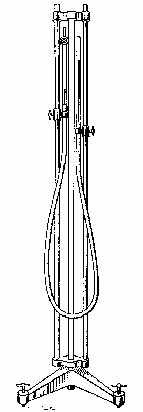 |
| Fig. 1. Mercury manometer. |
|---|
The apparatus is a manometer, consisting of two vertical glass tubes connected at their bottom ends by a length of rubber tubing. One of the glass tubes can be closed off at the top with a stopcock, thus confining some air or other gas in the tube. The other tube is open at the top, and may be moved up and down to adjust the mercury levels. Mercury fills the lower part of the tubes so that its levels are visible through the glass tubes. A metric scale between the tubes allows measurement of the mercury level in either tube.
The gas to be tested will be air; the sample is obtained as follows: Open the stopcock. Adjust the heights of the tubes so that the mercury level in the stopcock tube is about the midpoint of the glass tube. Close the stopcock, trapping an air sample in the tube. The pressure of that sample will then be atmospheric pressure, but this may be changed by moving the other tube up or down, compressing or expanding the air sample.
Even if the bore of the tube were known, it would be difficult to determine the volume of the gas sample because of the irregular shape of the stopcock. However, the changes in volume are proportional to the changes in length of the sample column, since the bore is uniform. So the equation PV = C can be written PL = C where C and C are different constants, and L is the length of the sample column. Experimental verification of either of these equations is a verification of Boyle's law, we will choose the latter equation.
The experimental data consists of simultaneous measurements of heights of the two mercury columns with respect to the metric scale. Take such data through the full range allowed by the apparatus. The pressure on the sample may then be calculated for each case. This pressure is sometimes above atmospheric, and sometimes below, so be careful of the signs of all quantities. Do not forget to add in the atmospheric pressure, and use consistent, absolute pressure units.
3. ANALYSIS:
(1) Plot P vs. L. What is the mathematical form of this curve?
(2) Plot P vs. 1/L. What is the mathematical form of this curve? Why? What is the significance of this graph?
(3) Which of your graphs most clearly shows whether you have verified Boyle's law? Why?
(4) Considering the general gas law, is it necessary to be concerned about error caused by room temperature fluctuations? If the room temperature changed by 5 K (perhaps someone opened a window) how much error would this introduce into your results?
Text © 1997, 2004 by Donald E. Simanek.