P-1 CALCULATION EXERCISES
Your textbook probably contains a discussion of the metric System, unit conversions, and exponential notation. Further discussion of these topics will be found in the appendices of this lab manual. The student should become familiar with this material before coming to lab.
A more detailed reference which some students may find helpful throughout the course is: Kruglak and Moore, Basic Mathematics for the Physical Sciences, McGraw-Hill, 1963.
2. PROCEDURE
This exercise is a problem session rather than a laboratory investigation. Its purpose is to make sure that all students can to do the fundamental mathematical manipulations required in this course. The problems are graded in difficulty, and should therefore be done in order. There are enough problems so that even the fastest student will be occupied most of the period. Do not rush to finish--be sure you understand each problem before proceeding to the next. If you are stumped on a problem, ask the instructor to discuses it with you, for he may give you some hints.
Feel free to discuss the problems with your lab partner, but share the work. To prevent confusion in the lab room, do not extend the discussions to other groups. We will follow this rule throughout the course.
3. MEASUREMENTS:
(1) If you are unfamiliar with metric length measurements, first examine a meter stick to learn the meaning of its markings. The meter stick is subdivided into 100 marked divisions called centimeters. Each centimeter is subdivided into 10 very small divisions called millimeters. What are the smallest divisions marked on the meter stick? How are the decimeters marked?
(2) Use a meter stick to measure the length and width of a lab table top. Devise some way to minimize the uncertainty due to the rounded edges of the table. Calculate the surface area of the table, and express this answer in three ways: square centimeters, square meters, and square millimeters.
(3) Devise a way to determine the thickness of the paper of a page of your textbook. How much uncertainty does this measurement have?
DISTANCES AND SIZES IN THE UNIVERSE
SIZE Object or distance. (in meters)
1028
1027
1026 - Distance from the sun to the most distant galaxies (1010 ly)**
1025
1024
1023
1022 - Distance to nearest galaxy (Andromeda) 2.15x106 ly
1021 - Diameter of a typical spiral galaxy (ours), 105 ly
1020
1019
1018
1017 - Distance from the sun to the nearest star, Alpha Centauri, 4 light years.
1016 - Distance light travels in one year, 9.46053x1015 m = 1 ly.
1015
1014
1013
1012 - Distance from Pluto to Sun, 5.9x1012 m
1011 - Distance from Earth to Sun, 1 AU = 1.496x1011 m
1010
109 - Diameter of the sun. (Equatorial radius = 6.9599x108 m)
108 - Diameter of Jupiter. (Equatorial radius = 7.19x107 m)
107 - Diameter of the Earth. (Equatorial radius = 6378.164 km)
106
105
104 - Height of Mount Everest, 8848 m = 28,028 ft
103
102 - Height of a tall building.
101 - Height of a two-story house.
1 - Height of a person (1.8 m)
10-1 - Size of a mouse
10-2
10-3 - Size of a flea.
10-4 - Size of a grain of sand.
10-5 - Size of a blood cell.
10-6 - Size of an animal virus.
10-7 - Size of a bacterial virus.
10-8 - Size of a large molecule.
10-9 - Size of a sugar molecule.
10-10 - Radius of a carbon atom, 10-10 m = 1 fermi (Fm)
10-11
10-12
10-13
10-14 - Radius of largest atomic nucleus, 10-14 m.
10-15 - Radius of the electron, "classical", 10-15 m.
* Adapted in part from Pauling, General Chemistry, 2nd Ed., Freeman, 1953,
Seeds, Horizons, 1981, and Burns, Modern Physics, 1988.
** The most distant objects identifiable as galaxies on photographs are about 10 billion light
years from Earth (Seeds, 1981).
Express all answers in exponential notation in standard form, rounded to the appropriate number of significant figures.
(1) Calculate the number of cubic centimeters in a cubic meter. (The answer is not 100.)
(2) A classroom has dimensions of 5.8 meters, 10.2 meters, and 3.5 meters. Calculate the volume of the room in cubic meters.
(3) Use the result of problem 2 to calculate the number of cubic centimeters in the room described in problem 1. Then check this answer by first converting the room dimensions to centimeters, then multiplying them together.
(4) How many cubic centimeters are in a cubic kilometer? Since this is a large number, express it in exponential notation.
(5) One light year is the distance light travels in one year. The speed of light is 2.9979250 x l08 meters per second. What is the length one light year in meters, to three significant figures.
PROBLEM SET A
(A1) For expressing very large numbers, the unit duo has been invented by mathematicians. A duo represents the number 1 followed by one hundred zeros. A still larger unit, the duoplex is defined to be 1 followed by a duo of zeros. Express both of these units in exponential notation in an unambiguous manner. All of the numbers used in physics happen to be smaller than a duo.
(A2) The radian is a unit of angular measure commonly used in science and engineering. The defining conversion relation between the radian and the degree is: 2p radians º 360°. The smaller an angle becomes the more nearly is its radian measure equal to its sine. So, for small angles, one can approximate the sine function by using the radian measure instead. How small must an angle be for this approximation to be correct to three significant figures? To four significant figures? (Use a digital calculator or consult a good trig table to answer this.)
(A3) Find the sine of 0.0001 degree to three significant figures. How much more precisely could you determine this?
 |
| The earth as a bowling-ball. |
|---|
(A4) An article about prime numbers in the Scientific American magazine of March, 1964 mentions that "The Computer Division of Los Alamos has a magnetic tape on which 20 million prime numbers are recorded." Suppose we wanted to print all of these numbers in a book. A moderate speed computer printer can print about 200 lines per minute. If one prime number were printed per line, how long would it take to print all those prime numbers? Express the answer in days, or years, whichever is more appropriate. If the printout has 60 numbers per sheet of paper, how thick would be the stack of sheets? If reproductions were made and sold at typical "textbook" prices, how much would a copy cost?
(A5) If we made a scale model of the earth the size of a bowling ball, how high would Mount Everest be on the model, in inches? In cm? The earth's radius is 6.4 x 106 meters; Mount Everest is 8.85 km high. Regulation bowling balls must not have a circumference greater than 27 inches. (Sports have not yet gone metric in this country.)
PROBLEM SET B
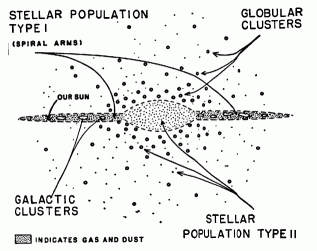 |
| Structure of our Galaxy (edge view). |
|---|
(Bl) The estimated amount of hydrogen in interstellar space is one atom per cubic centimeter. Our galaxy is shaped somewhat like a round disk, with a diameter of 300,000 light years and a thickness of 35,000 light years. Approximately how many atoms of interstellar hydrogen are there in our galaxy? (The data is only accurate to one significant figure, so express the answer accordingly.)
(B2) What is the mass of one cubic centimeter of water at a temperature of 20°C? [Look this up in a reference book.] In elementary chemistry and physics lab work this is often taken to be approximately 1 gm/cm3. What percent discrepancy will result from use of this approximate value? Will this be significant in our work?.
(B3) Masses of atoms are measured using a unit called the unified atomic mass (u) defined so that the mass of the isotope Carbon-12 (12C) is exactly 12u. As a consequence, one u represents a mass of 1.660559 x 10-24 gm. The mass of an atom of iron is 55.934939 u. What is the mass of an iron atom expressed in grams? How many atoms are there in a gram of iron? Express both answers to 3 significant figures, using the rules of chapter 1 of An Introduction to Experimental Analysis.
 |
| Flea (Ctenocephalides canis). |
|---|
(B4) A flea can jump over a foot upward. If you could jump that well, relative to your size, how high could you jump?
(B5) The theory of the moon according to the analytic methods of Charles Delaunay (1816-1872) contains one equation exceeding 170 pages in length. If the pages were the typical size of books of that era (9 x 6 inches), and laid side-by side in the hallway, how many feet long would the hallway have to be?
PROBLEM SET C:
(C1) In the chart on page 3 of this experiment, the distance to the farthest known galaxy is shown. Check the consistency of this value with the value given in the footnote on page 2.
(C2) The unit of mass in the metric system was chosen so that the density of water would be 1 gm/cm3. The density of water changes slightly with temperature, but for most calculations this change is small enough to neglect. At-room temperature (20°C) the density of water is actually O.99823 gm/cm3. What percent discrepancy will result from using 1 gm/cm3 at this temperature instead of the more correct value? Will this be significant in our work? Calculate the correct volume (in cm3) of 1000 grams of water at 20°C.
 |
| Pretty picture of atom as seen in some textbooks. [Not to scale.] |
|---|
(C3) If you drew a "picture" of an atom one inch in diameter, how large would you have to draw the nucleus? Compare your conclusion with pictures of atoms you have seen in textbooks. Consider drawing a scale model of the solar system with the orbit of Pluto five inches in diameter. How large would the sun be on this picture? Compare with the pictures you have seen.
(C4) In elementary science books, the atom is often described as a "miniature solar system." Consult the chart of page 3 of this experiment along with the results of problem C3 to determine whether this statement is an accurate analogy, at least with respect to the sizes of the component parts of the system.
(C5) The German philosopher Friedrich Wilhelm Nietzsche (1844-1900) said "The earth has a skin and that skin has diseases; one of its diseases is called man." Physicist Albert Abraham Michelson (1852-1931) told his students (at the University of Chicago) that mankind as a whole was rather insignificant on a universal view, merely "a skin disease upon the face of the earth." Consult the chart of sizes to determine whether this statement is an accurate comparison of relative sizes.
 |
| Mankind: a crawling disease on the face of the earth. Drawing by John C. Holden. |
|---|
PROBLEM SET D: (homework)
(D1) Gerald Holton, Derek J. de Solla Price end others have estimated that "80 to 90 per cent of all scientists that have ever been, are alive now." Should this surprise anyone? How would this ratio compare with other professions? With the general population? Discuss, citing appropriate data from library research.
(D2) Theodor Rosebury, in his fascinating book Life on Man (Viking Press 1969, Berkeley paperback 1970) estimates that the normal healthy skin of a person has about 1011 microbes. Estimate the total skin surface of a typical person and calculate the average number of microbes/cm2. How much area does each microbe have to itself, on the average? The human population of the earth is now over seven billion persons. How much land area does each person have, on the average? Make a meaningful comparison between the surface population of people on earth, and microbes on man. Don't merely compare the number/area ratios for the two cases, consider also the sizes of microbes and men.
(D3) (a) Find out the top speed of a garden snail moving across the ground in search of juicy leaves to munch on (a snail's-pace). (b) Express this snail's pace in centimeters per second. (c) Express this also in the units furlongs/fortnight.
Text © 1997, 2004 by Donald E. Simanek.