H-2 SPECIFIC HEAT CAPACITY
To accurately determine the specific heat capacity of a metal, taking care in experiment design to eliminate sources of random and systematic error.
2. APPARATUS
Steam generator (hypsometer) with stand.
Bunsen burner, or electrical heater.
Beaker to catch drips from condensing steam.
Shot cup, cotton.
Double wall calorimeter with stirrer.
Brass, iron, copper or aluminum shot.
0 to 50° C limited range thermometer, least count 0.1°.
Clip-on thermometer magnifier.
Single hole cork to fit thermometer and calorimeter.
Sheet of Cartesian graph paper.
3. THEORY
(1) HEAT. When an amount of energy is transferred from one body to another solely as a result of the temperature differences between the bodies, we call that amount of energy "heat." Bodies can transfer energy in this way through three processes: radiation, conduction, or convection. When, due to the heat transfer, the hotter body loses thermal energy and a cooler one gains that same amount of thermal energy, the process continues until both bodies reach the same "equilibrium" temperature.
In the 18th century scientists thought that heat was something like a fluid that "flowed" from one body to another. Though this model has long been abandoned, much of our terminology still carries remnants of this archaic idea. Some books still say "heat is transferred from one body to another." This should be understood as a shorthand way of saying "energy is transferred from one body to another through a thermal process." One should not say there is "heat in a body." We say instead that the heat given to a body raises that body's internal thermal energy. This usage is similar to the way we use the word work. We say that body A does work on body B, but we do not say that this work was "in body A." Both "work" and "heat " are words that describe and measure the amount of energy transferred from one body to another, but we use other names to represent energy in or possessed by a body.
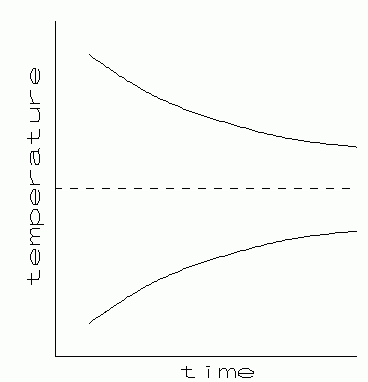 |
| Fig. 1. Cooling and warming curves. |
|---|
| [1] |
(d/dt)ΔTo = - K ΔT
If at some time t = 0 the temperature difference is To, then at a later time t the temperature difference will be:
| [2] |
ΔTo = ΔT e-KT
This can be shown by integrating equation 1.
Therefore, when two bodies exchange energy thermally, the temperature of each will exponentially approach their common final equilibrium temperature.
(3) SPECIFIC HEAT CAPACITY. When a body gains or loses energy in a thermal process, we find experimentally that the temperature change of the body is proportional to its change in energy:
| [3] |
ΔH = ms(ΔT)
where H is the amount of energy (the "heat") gained or lost in the thermal process. The mass of the body is m and the quantity s is a thermal property of the body called its specific heat capacity, usually shortened to specific heat. (While the latter shortened from is easier to say, it can be misleading. This quantity is not a form of energy or heat, and does not have the units or dimensions of heat.)
(4) HEAT UNITS
The kilocalorie (kc) is defined to be the amount of heat required to increase the temperature of 1 kilogram of water 1° C. In nutrition studies this is simply called the "calorie." In physics books that use the cgs system the gram calorie (gc), or small calorie is defined as the amount of heat required to increase the temperature of 1 gm of water 1° C. In those books it is simply called the "calorie". So when you see the word "calorie" look at the context and be sure you know whether it means the small or large calorie. In both systems, the specific heat capacity of water is taken to have size 1.
The British thermal unit, (BTU) is defined as the amount of heat required to increase the temperature of 1 pound of water 1° F. In this system the specific heat capacity of water has size 1.
(5) CALORIMETRY. Consider two bodies at different temperatures, insulated from all other bodies, yet able to transfer heat to each other. The heat gained by the initially cooler body is equal to the heat lost by the initially hotter one. Then equation (3) applied to each body gives:
| [4] |
m1s1 (ΔT)1 = m2 s2 (ΔT)2
This situation, and this equation, are the basis of a class of experimental methods called "calorimetry." Calorimetry allows us to measure the ratio of specific heat capacities of any pair of bodies. Water is taken as the standard, setting its specific heat capacity to be numerically equal to 1, in both cgs and mks systems.
The specific heat capacity of water is taken to be 1 small calorie per gram per Celsius in cgs; and 1 kilogram per Celsius in mks. Calorimetry experiments were being done before anyone was able to express or measure heats in the "mechanical" units of ergs and Joules. Therefore the calorie units were defined as the amount of heat required to raise one unit of mass on degree, in each system. In nutrition studies, the amount of energy available in foods is expressed in kilocalories, but as that is the only kind of calorie they use, they have dropped the prefix kilo.
Of the available heat transfer processes, conduction usually provides the most rapid heat transfer. That's why it is used in many calorimetry experiments.
One difficulty in calorimetry is insulating the experiment to prevent heat transfer to or from the surroundings. The water in the experiment is in a container, and the container will exchange heat with the water. So will the thermometer.
(6) WATER EQUIVALENT. For a given volume of material, the effectiveness of the material for producing a temperature change is proportional to its water equivalent, the product of its mass and its specific heat capacity.
4. EXPERIMENTAL STRATEGY:
(Read this entire section before beginning the experimental work.)
The "unknown" material is in the form of metal particles or "shot." This is heated to steam temperature, then dumped into cool water in the calorimeter and allowed to come to an equilibrium temperature. The necessary measurements are: mass of water, mw; initial water temperature, Tw; mass of metal sample, ms; sample temperature, Ts; and the final equilibrium temperature of the mixture, Tf. The heat exchange equation is:
| [5] |
mw(Tw - Tf) + msss(Ts - Tf) + mcsc(Tc - Tf ) = 0
The subscript c labels quantities that describe the calorimeter inner cup. This cup also participates in the heat exchange. Its temperature is nearly the same as the water in it, so Tc = Tw.
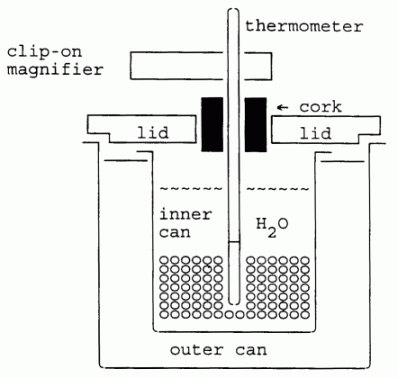 |
| Fig. 2. Double-walled calorimeter, assembled. |
|---|
Several kinds of calorimeter may be available:
(1) A styrofoam cup with a styrofoam lid. This plastic material contains many cells of trapped air and is a remarkably effective insulator.
(2) The double-walled calorimeter, which consists of two metal cans, usually aluminum, separated by an insulating fibre ring at the top, and covered with a wooden or plastic lid. The trapped air between the cans provides insulation. This space may also be filled with fiberglass.
(3) The vacuum calorimeter is a Dewar vacuum flask (similar to a Thermos (TM) bottle). The inside wall is silvered to prevent radiative transfer to the outside; the vacuum prevents conduction.
(4) The "adiabatic wall" container in which the walls are kept at the same temperature as the contents inside. This is accomplished by temperature sensors, electrical heaters, and an electrical feedback system that senses any slight temperature difference and quickly corrects it.
When the first two kinds of calorimeter are used, it is important to prevent heat transfer through the container from affecting the experimental results. A standard way to do this is the "method of compensated loss (or gain)." The idea is simple: start with the water below room temperature and end with it about the same amount above room temperature. This ensures that the heat gain from the environment in the early stages of the experiment is balanced by a nearly equal heat loss in the later stages. To achieve this will require some knowledge of the expected temperature change, so the initial water temperature is chosen correctly. A preliminary "trial run" can give this information.
This is sometimes called "Rumford's method" after physicist Benjamin Thompson (1753-1814) who was given the title "Count Rumford" while he was in the employ of the Emperor of Bavaria. During this time Rumford made many studies of heat, the "force" of gunpowder, insulating properties of clothing, and even efficient design of cookstoves. From these studies he reached the conclusion that heat had no mass and was not a substance. He speculated that heat represented some kind of "motion" within materials. Though he did not carry this iedea further, he was on the right track. We now recognize that the thermal energy content of a substance is the total kinetic energy of motion of the particles within it.
Insert an accurate thermometer in the calorimeter to monitor the temperature of the water in the inner cup. Take care that the thermometer is inserted to the proper depth. Most thermometers have a line engraved around the stem, indicating the exact depth to which the thermometer should be immersed in liquid to insure accurate temperature values.
Some calorimeters have a stirring plunger. While temperature readings are being made the water should be gently and continually stirred. Move the plunger up and down with slow and regular strokes. Maintain uniform stirring both before and after adding the shot. Avoid stirring too vigorously, for that can add significant mechanical energy to the system!
One still must consider the heat transfer to the inner cup and the thermometer. Both are in contact with the water and can exchange heat with it. The "compensated loss" procedure only corrected for heat exchange with the outer environment, but did nothing to correct for heat exchanges within the calorimeter.
We know the materials of the cup and thermometer (aluminum and glass). We can weigh the cup, and can estimate the fraction of the glass of the thermometer that participates in heat exchange with the water. Then, using handbook values of the specific heat capacities of aluminum and glass we can calculate the size of the terms that contribute in the heat exchange equations.
| [6] |
mcsc(Tw - Tf) + mtst(Tw - Tf)
Since these bodies follow the water temperature, they share the (Tw - Tf) factor with the water term of the heat transfer equation. We can therefore combine all three terms:
| [7] |
(mw + mcsc + mtst)(Tw - Tf)
The quantity (mssc + mtst) = meq is often called the "water equivalent" of the cup and thermometer. Once it is determined, this number is simply added to the measured mass of water, for the purposes of calculation of the heat transfer equation:
| [8] |
(mw + meq)(T2 - Tf) + msss(Ts - Tf) = 0
The water equivalent correction will be small, and need not be of high accuracy unless the temperature differences can be measured with high accuracy. The error analysis will show how accurate this must be.
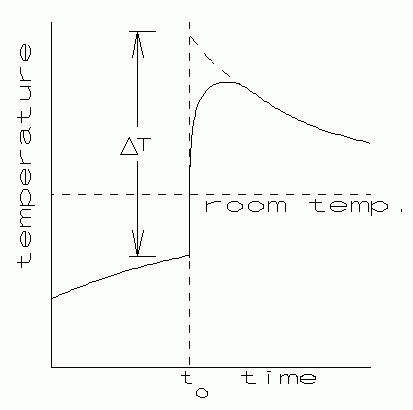 |
| Fig. 3. Method of temperature correction. |
|---|
The final experimental difficulty to be overcome is the thermal "sluggishness" of the thermometer. If you watched carefully as the hot metal was added to the water, you noticed that the thermometer reading did not change immediately. It took time for the thermal energy to transfer to the thermometer fluid. By the time the thermometer reached equilibrium with the water, the water had already cooled somewhat by energy loss to the surroundings. This "thermal lag" error of the thermometer might cause the calculated final temperature to be too low.
The error analysis will show that the experimental error is mostly due to error in the temperature measurements.
With typical data, and temperatures accurate to 0.5° C, the temperature uncertainties will contribute at least 6% to the uncertainty of the results.
One can correct for thermometer sluggishness in the following way. Monitor the water temperature at half-minute intervals for about 5 minutes before the hot metal is added, and also for about 5 minutes after it is added. When this temperature data is graphed against time, it may look something like Fig. 3. The "Newtonian" cooling and warming curves are evident, and can be extrapolated back to the time the shot was dumped in.
If the temperature measurements are very accurate, you may need to determine the water equivalent by a better method. One way is to perform a separate experiment in which hot water is added to cold water in the calorimeter in such amounts as to duplicate the temperature change of the original experiment. You also must duplicate the initial and final water levels of the original experiment&151;in short, keep everything as much the same in the two experiments as possible, except this time you use no metal, just hot and cold water. Now the heat-transfer equation is"
| [9] |
(mcw + meq)(Tcw - Tf) + mhw(Thw -Tf) = 0
where "cw" and "hw" label cold water and hot water.
5. PROCEDURE
(1) Weigh the inner cup of the calorimeter, and weigh the thermometer. Note whether the specific heat values of the calorimeter cup is stamped on it. If it is not, determine the metal of which it is made, and estimate its specific heat value.
(2) Determine what mass of water would be required to fill the inner cup 2/3 full.
(3) Do a trial calculation to determine what mass of shot at 100° C must be added to cold water at 5° below room temperature to give a final equilibrium temperature about 5° above room temperature. Since you don't yet know the specific heat of the metal, you will have to make an intelligent guess of its value for this calculation. Consult handbook values for metals, but keep in mind the possibility that the sample you have been given just might be an alloy whose value is not in the tables. We want to achieve a temperature change of at least 10° in water, without using so much shot that the cup becomes overfull.
(4) Now that you have determined how much shot to use, weigh out that much. Put it in the steam-generator cup, plug the top of the cup with cotton, and heat the shot in the steam- generator.
(5) Put the cold water in the calorimeter inner cup and weigh both together. Assemble the calorimeter and begin monitoring the water temperature every 1/2 minute. Use the limited range (0-50° C) thermometer here, with the clip-on magnifier, for accurate readings.
(6) When the shot cup has been in the steam bath for at least 5 minutes the metal shot will be at the steam temperature (near 100° C depending on the barometric pressure). Quickly transfer the shot into the calorimeter and reassemble it, then continue your temperature readings every of the water in the calorimeter every 1/2 minute. The exact time of dumping in the shot must be noted also. Take temperature readings for long enough to clearly establish the cooling curve of the water.
For more precise determination of the steam temperature, read the barometric pressure in the laboratory and consult the tables in the Handbook of Chemistry and Physics that relate boiling point to pressure.
(7) At the conclusion of the experiment, put the wet shot in the designated place to be dried. Do not put wet shot back in the original container. Empty the water from the boiler and the calorimeter. Dry any spills on the table and floor.
6. ERROR ANALYSIS
(1) Solve the heat-exchange equation algebraically for the specific heat of the unknown sample. Include the calorimeter cup's contribution to the heat exchange.
This is an example of a situation where there's very little to be gained from deriving a complete error equation. Much of the analysis of errors can be carried out in a common-sense manner as suggested below. Your discussion should include, but not be limited to, consideration of the following questions.
(2) Compare the errors in the specific heat determination that would be caused by (a) a 0.1° error in measuring the temperature change of the water, and (b) a 0.1° error in determining the temperature change of the metal sample.
(3) Suppose you had neglected to include the term representing the calorimeter inner cup. What percent change would this have made in the calculated value of the specific heat of the specimen? Would the specific heat value have been too high, or too low? How would this systematic error compare with the other experimental errors?
(4) If all other sources of error were negligible, how accurate would the temperature measurements have to be to ensure a 2% accuracy in the determination of the specific heat of the metal?
7. QUESTIONS
(1) The British Thermal Unit of specific heat capacity, (BTU), still used by some engineers, is defined to be the amount of heat required to raise one pound of water one degree Fahrenheit. That is, ΔH = WsΔT, where W is the weight in pounds, H is in calories, T is in degrees Fahrenheit and s is the specific heat capacity in BTU. The specific heat capacity of water is taken to be numerically equal to one. Use these facts, and Eq. 3, to derive the conversion equation relating the BTU to the kilocalorie.
(2) What are the units of specific heat capacity in (a) the cgs system; (b) the mks system; (c) the British system?
(3) Sometimes when a cold thermometer is immersed in a warm liquid, the mercury column will be observed to drop briefly, then rise. Develop a hypothesis suggesting why this might happen.
Historical note. This experiment is sometimems called "Rumford's method" after physicist Benjamin Thompson (1753-1814), who was given the title "Count Rumford" while he was in the employ of the Emperor of Bavaria. During this time Rumford made many studies of head, the "force" of gunpowder, insulating properties of clothing, and even efficient design of cookstoves. From these studies he reached the conclusion that heat had no mass and was not a material substance. He speculated that heat represented some kind of motion. Though he did not carry this idea farther, he was on the right track. We now recognize that the thermal energy content of a substance is the total kinetic energy of the particles within it.
Text and diagrams © 1996, 2004 by Donald E. Simanek.