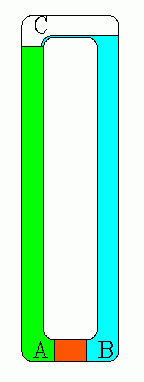The reverse osmosis problem.
Simple answer
The nice thing about this problem is that you don't need to know how osmosis or reverse osmosis work at the molecular level. The given information is enough.The deception is partly in the wording of the problem, and the description that supposes that you lower the tube already filled with fresh water. If the tube were filled with water up to the ocean surface, the pressure difference across the plug would be much too small to initiate reverse osmosis.
 |
To initiate reverse osmosis the pressure across the membrane or semi-permeable plug must be approximately 20 atm. The pressure at a depth of 200 meters below sea level is about 20 atmospheres greater than atmospheric pressure. These approximate values will serve our present purposes.
So, to initiate reverse osmosis across the membrane, the fresh water in the tube must be at least 200 meters lower than the surface of water outside the tube, to have a 20 atmosphere pressure difference across the membrane. This is true no matter how long the tube is or how deep it goes into the ocean. End of story. The idea will not work. It's that simple.
What's amazing is how easily people become distracted by irrelevant considerations, water salinity, stratification of salinity density, engineering difficulties of making a long pipe, necessity of cleaning the filter membrane, economic considerations, storms at sea, etc. etc. They are so obsessed with what they see as "practical" considerations that they are blinded to the simple logical flaw that really does make the idea unworkable. For an example of this, see: a web discussion. Hearing these people talking all around the issue as if they knew something about it, and then still missing the point, is quite instructive. Go to any of the internet discussion groups devoted to perpetual motion and you will see the same sort of uninformed pseudo-profundity in abundance.
But still, one must be careful of the assumptions used to reach a conclusion. In this case we assumed that the density of fresh and salt water were the same. That's actually not true. Does it matter?
Details
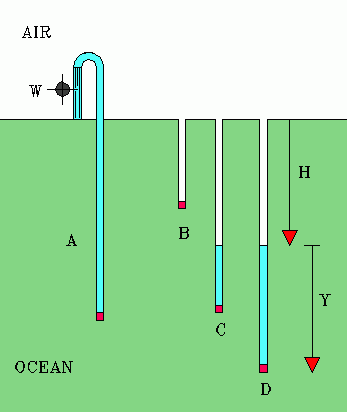
Of course, consideration of the details can be interesting in itself. So here's more about this classic problem.Figure A illustrates what the optimist hopes will happen. The tube has a semi-permeable plug (red) at the bottom, lowered to a depth at which the water pressure of the ocean is sufficient to make reverse osmosis happen. Fresh water gushes from the top of the tube, turning a small waterwheel, W. Figures B, C, and D illustrate what really happens.
Fig. B. The tube does not reach to sufficient depth. Nothing happens.
If we lower the tube with the semi-permeable plug to a depth of H = 200 meter where the water pressure outside the bottom of the tube is 21 atmospheres. But the pressure inside the tube is only 1 atm. The pressure across the porous plug is 20 atmospheres. Still, nothing happens, for any water through the plug would create pressure above and stop the process.
Fig. C. Now suppose we lower the semi-permeable plug to greater depths. At a depth of 300 meter the pressure difference across the semi-permeable plug is initially 30 atmospheres, more than enough for reverse osmosis. Pure water rises inside the tube, and this column of water exerts pressure on the top of the plug, so the pressure difference across the plug gets smaller and smaller as the water inside the tube rises. The water inside the tube rises until it is 100 meter above the porous plug and exerts an 11 atmospheres pressure on the top of the plug. The pressure difference across the porous plug is 31-11 = 20 atmospheres. Reverse osmosis stops. The water level inside the tube is 200 meters below sea level. Not good enough.
Fig D. Now lower the plug to a depth of H+Y = 400 meter. The ocean pressure at this depth is 41 atmospheres. The pressure on the top of the plug is initially 1 atmospheres. Pure water rises inside the tube until it exerts a pressure of 21 atmospheres on the plug. The pressure difference across the plug is only 41-21 = 20 atmospheres again. Reverse osmosis now stops. The fresh water inside the tube has not reached sea level, in fact it is again 200 meters below sea level.
That 1 atmospheres pressure goes along for the ride in the calculations above, since it affects pressure on both sides of the plug equally. I was tempted to leave it out, but then someone might think that its omission made a difference in the results. It doesn't.
An easier way to see this is to note that there must be a minimum of 20 atmospheres pressure difference across the plug. So, when static equilibrium is reached, at any depth, the liquid inside the tube must exert on the plug 20 atmospheres lower pressure than does the water outside. Therefore the top of the water column inside the tube must be 200 meters lower than sea level, since a 200 meter high column of water exerts a pressure of 20 atmospheres.So no matter how deep you lower the porous plug, the water inside will rise only to a height where it is 200 meters below sea level, then stop. Our hopes of infinite energy and unlimited fresh water are dashed.
Some may quibble that we haven't taken into account the 2.5% difference in densities of salt and seawater. Or some may note that we haven't considered changes in salinity with depth, or some other detail. We explore that objection in the next section.
Thanks to Roy Havenhill for useful and perceptive comments and suggestions for improvement of this document.
The 2 1/2 percent solution.
[January, 2007.] Over the nearly three years this problem has been sitting here, I've gotten emails every few months from people who point out to me that the 2.5% difference in density between salt and fresh water has not been taken into account in my "simple solution". They may not have noticed that I acknowledged this in the next to last paragraph of that section, subtly inviting readers to provide the solution to that puzzle. In every solution lurks another puzzle. I must admit that I "put off" these folks, giving only a few hints for how that might be done. Some left, disappointed, and perhaps some were angry because I didn't provide "the answer". I've always thought that answers are less interesting than the process for getting answers.Hints that I gave included:
- The question was not about whether reverse osmosis works (it does) but whether you could use it to make a perpetually flowing cycle and extract useful work from it without causing the flow to slow and stop.
- Perpetual motion of a cyclic process requires that the process remain in motion and perhaps also produce excess work. So, in this device, we must consider fluid flowing continuously forever.
- The simple solution given above was a static solution. Perhaps a dynamic solution is necessary.
- Perhaps enlightment would come from devising a simple conceptual experiment to find out how much work it takes to dissociate salt from salt water to produce fresh water. No one took me up on this.
- The pipe isn't a closed system, for it sits in and can interact with the entire ocean. If we created a closed system, would this work the same way?
- When the pipe and plug is lowered into the ocean, work is done displacing a column of sea water. This represents potential energy. This is recovered as the fresh water rises to its equilibrium position in the pipe. In effect, the system starts already "primed" with energy. So the initial rise does not represent energy "from the ocean".
Quite a number of people presented the following analysis, extending my simple solution. This shows that with sufficient depth, the system will support a column of fresh water that is higher than sea level. This agrees with calculation I had previously done, but not revealed on my web page.
Since seawater is 2.5% denser than fresh water, then the column of fresh water inside the tube is 2.5% longer than I calculated above. So when the porous plug is 40 meter below the surface, the water inside is not 200 meters below the surface, but 200 - 200(0.025) = 0.975(200) = 195 meters. So for every additional 200 meters we lower the porous plug, we get the fresh water 0.5 meters closer to the surface. 200/5 = 40, and if we lower the plug to (40+1)(200) = 8200 meters = 8.2 km, the fresh water level inside the tube would be at sea level. Lower the pipe and plug still more and you'd have the fresh water surface above sea level and could then let it overflow to produce fresh water and useful work for free.The greatest depth in the ocean, the Marianas trench, is about 11 km, so ocean depth isn't a limitation!
Can we then use this to create a cyclic perpetually sustained process from which we can extract energy and fresh water? We suspect this analysis can't be right. As I've said elsewhere on these pages, the basic and well-tested laws of nature, like Newton's laws, conservation laws, and laws of thermodynamics crucially depend on an even more fundamental law, the principle of Perpetuum immobile, or "Nature abhors perpetual motion." (Nature cannot produce perpetual motion.) Whenever our analysis of a physical system concludes that it might allow perpetual motion or over-unity performance, then we know that we should look for one of the following errors in our analysis:
- We have blundered in a calculation.
- We have overlooked some process in the system.
- We have overlooked some source of energy input.
- We have overlooked energy initially present in the system.
- We have done the calculations of power output or input incorrectly.
- We have measured the power output or input with inappropriate instruments or improper methods.
But here I must still leave this one remaining problem to the readers, for I havn't a good answer to this messy problem.
[Dec 18, 2007] A note to those who have been knocking this problem around on a message board recently. I am fully aware that this device is not perpetual motion, and certainly isn't an "over-unity" device, for I'm convinced that such devices are not possible in nature. Why is this puzzle here at all? Because when Derek Christie in New Zealand directed my attention to it in Scientific American I realized that, as it was presented there, it was a clever deception, and certainly qualified as "unworkable". But, when you take into account the density differences between salt and fresh water, and make the pipe long enough, it seems that it could "pump" water continually, so it becomes a bit perplexing. The analysis in Scientific American seemed incomplete and unsatisfactory to me.
Frankly I was sorry I got into this, for I don't care for such messy problems. They can become an obsession. So I've left it alone, suspecting that there's some simple resolution that I'm not seeing because I'm looking in the wrong place or using an inappropriate approach. I was hoping someone else might "see it".
|
Here are some things that one might try:
- Remove the complication of the larger ocean and consider a closed loop made of two very (sufficiently) long pipes connected at the bottom with the porous plug and at the top by an inverted "U". (See the picture.) No energy from outside is allowed into the tube. Will water flow continuously around this loop? Using the same arguments that we used above, it seems it should, and that's the problem, for that would indeed be perpetual motion and that's simply not possible.
- Look into the matters we ignored above. The water is not static, but flowing, at least until it stops. Water flowing through the plug loses energy due to the pressure difference across the plug. Usually we ignore friction as a first step in analyzing these unworkable machines, but here we cannot get away with that strategy. Why? Because our previous analysis (which got us into trouble) did use the fact of the pressure difference across the porous plug, so we must consistently use it wherever it has an effect. If there really were no friction, there'd be no reverse osmosis effects, and the whole problem would be pointless. We must admit the pressure difference is there, so we must also admit the energy loss as water is forced across that pressure difference.
- Finally, I think it would be instructive to consider the simple (!) problem of the energy required to remove salt from water. The salt doesn't separate out by itself. We lose system energy as the system removes the salt. So consider a cylindrical tube of salt water sitting on a table. Now "magically" remove the salt and place it on the table nearby. If the table is taken to be "zero" for calculation of potential energy, the salt that was in the tube had potential energy of ½rswgh, where h is the height of water in the cylinder. After salt removal, the remaining water is the cylinder is fresh, and has a greater height, H. Its potential energy is ½rwgH. Now compare the potential energy of the system before and after. Now our reverse osmosis tube is different. The salt is removed physically by the plug within the system, and there's energy required to do that removal, which has to come from somewhere in the system.
Before one gets excited about the practical possibilities of getting useful work for free from this device, consider this fact derivable from thermodynamics: "0.66 kcal / liter is the minimum energy required for desalination of one liter of seawater, regardless of the technology applied to the process..." This calculation assumes a reversible process and perfect efficiency. "Practical desalination systems are never fully reversible and there are energy losses that are due to unavoidable irreversible contributions. These losses, that depend on the water recovery ratio, increase the energy [required for] desalination above the reversible thermodynamic limit." These quotes are from Energy of Seawater Desalination by Uri Lachish. Note: 0.66 kilocalorie/liter is 0.767 kilowatt-hr/m3. But the best reverse osmosis desalination systems are far less efficient than the thermodyamic ideal, requiring more like 5 kilowatt-hour/m3. - Then there's the little overlooked matter of what happens at the top. If the fresh water did rise a bit above the ocean surface, and was redirected over to the salt water tube, that fresh water would gradually decrease the salinity in the salt water tube. As one correspondent suggests, this might continue until all the water in the system was fresh. How long might that take? But if we are using a closed system isolated from outside energy sources, we must ask, "Where did the energy come from to maintain flow?" I am confident this scenario will not happen, for it ignores the large continual energy loss as water flows through the porous plug.
Indeed, I'm convinced that it is energy loss of water forced through the plug that dooms this device to failure. Construct a sufficiently long U-tube with porous plug at the bottom, and fill it with salt water on one side and fresh water on the other. Now these columns of water would be in static equilibrium with the fresh water higher than the salt water by 2.5%. That is the simple fact that seduced us into this mess.
But could that same result be obtained if the fresh water side were initially empty and then allowed to fill from pressure difference and reverse osmosis? The two cases are not the same energetically. In the static case we didn't lose energy dissociating salt from water. Also, the static case did not lose energy as water is forced through the porous plug. For both of these reasons the height on the fresh water side would end up lower than on the salt water side. And extra pipe length won't help.
The Final Solution?
[Feb, 2010] Let's dispose once and for all of the notion that you can get perpetual energy out of this system. Suppose you had an ocean deep enough that the fresh water would rise in the tube above the salt water ocean surface. To test this, get a very long tube, put a porous plug in the bottom, and also a cap so the water doesn't begin to flow through the plug right away. Lower it (slowly) into the ocean. In the process it displaces its own volume of seawater. Due to the buoyancy of the empty tube, you must do work on the tube as you lower it. This is because the tube pushes salt water aside and therefore raises the level of the ocean ever so slightly. When the tube is at the required depth, open the cap and let the reverse osmosis begin. Fresh water rises in the tube until flow stops. Now let's compare the energy of the initial and final states. The tube system (tube and ocean) had initial potential energy equal to the work you did when lowering it. At the end, the ocean energy has lowered slightly, but the fresh water column gains potential energy as it rises. But the water inside is fresh, and the salt that was in that volume of water has been left outside the bottom of the tube. It has lower potential energy than when that salt was distributed from that depth up to the ocean surface. When you sum the energies before and after you get zero, assuming an idealized reverse osmosis process (which is never even closely achieved in practice.)A kind reader, Glen Robinson, has done the work I was too lazy to do, and I quote his email in full.
Hi Donald,Dare I assume that this will be the final word on this subject? We have certainly exhausted the details of this problem, which began its life as a deceptive puzzle. I think we can safely conclude that (1) The idea is not an over-unity device, (2) nor is it even a practical method for extracting energy from the underwater enviroment.I’ve enjoyed reading your Museum of Unworkable Devices. Having read the Reverse Osmosis problem, it appears that you didn’t end up with a solid reason (or at least one that you were satisfied with) as to why it wouldn’t work when the 2.5% density difference is taken into account.
The answer is that theoretically, it would work as described, however not on earth. The minimum hydrostatic pressure needed to separate out the salt is 27.8bar (2.82MPa—assuming that the temperature at the deepest part of the ocean is 3°C). Using this figure instead of 20 bar, the minimum depth required is 11,502m, approximately 500m deeper than the Challenger Deep in the Mariana Trench. At this exact depth, the flow rate through the membrane would slow to zero at the point that the fresh water made it back to the surface, requiring that a “workable” system (one where there was reasonable flow) would need to be quite a bit deeper.
That said, the theory is sound—the energy in the system comes from the potential energy difference between having the mass of the salt at the top of the ocean to the bottom of the ocean. 25kg of salt (the difference in mass between 1m3 of salt and fresh water) moved 11502m can supply 2.82MJ – which exactly matches the work needed to push a 1m2 piston a distance of 1m (hence moving 1m3 of water) through a 1m2 membrane requiring a pressure of 2.82MPa. Additionally, the system wouldn’t need to have much excess pressure (as it does in commercial reverse osmosis systems), since the salt wouldn’t concentrate around the membrane during the process (increasing concentration increases the force needed to separate it) as the salt it would diffuse back into the surrounding salt water.
The depth issue can potentially be overcome in two ways: one, adding pressure to the salt side, or two, removing pressure from the fresh side.
One: by adding a comparatively low pressure pump (compared to normal RO system requirements) at the bottom of the pipe, however this then adds the issue of increasing the salt concentration (since the membrane will now have to be enclosed), necessitating either an increased flow rate (with lower pressure) so that the brine concentration stays low, or higher pressure to deal with the increased concentration. It loses the automatic remixing that the open system would have and also adds moving parts and a significant electrical equipment and power supply that you need to be able to keep very high pressure water out of.
Two: a much easier approach would be to pump water out of the fresh water pipe, keeping the water level down at say, 100m below the surface. It would then take approximately 1MJ/m3 (theoretical) to lift the water up to the surface. Losses in the pumping process would probably increase this to 1.5MJ/m3, however compared to world’s best practice RO at ~7.2MJ/m3, this is pretty fine indeed.
This is where the economics and other realities of the situation start to bite—the cost of an extremely deep pipe would be prohibitive and the requirement that there is very deep water available reduces the potential locations where it can be done. An alternative is where the pipe isn’t as deep, which would require a much greater amount of energy to lift the water. At minimum depth (~300m) the lift energy is only just a little less than the energy needed by the best practice RO systems, and flow rate would be low. You would then have to go deeper to get more flow, giving a greater lift energy required, making it marginal at best in terms of energy use. Also, the number of locations where relatively deep water (>500m) is close to a major population centre is limited. Finally, imagine the cost and complexity of having to clean and/or replace the membrane periodically.
It’s an “almost” thing, but not close enough.
Cheers,
Glen Robinson BAppSc (Physics), BEng (Elec)
—Donald Simanek
Document begun in 2004. Last modified Feb. 2010.
Return to The Museum of Unworkable Devices Annex.